Ready for Serialization?
Last Reviewed October 2023
The Drug Supply Chain Security Act (DSCSA) establishes a system for the tracking and tracing of prescription drugs through the supply chain and places new requirements on manufacturers, repackagers, wholesale distributors, dispensers and third-party logistics providers for complying with this system. The DSCSA sets forth a timeline in which the requirements under the law must be phased in. Some of these deadlines have passed, but critical requirements for wholesale distributors, repackagers, and manufacturers are approaching.
Things To Know For November 27, 2018 (Serialization Deadline):
- Manufacturers and repackagers are required to begin affixing or imprinting a product identifier (PI) to each package (smallest saleable unit) and homogenous case of a product intended to be introduced via transaction into commerce
- The PI must be printed with a machine readable barcode and human readable text
- The serial data record must be kept for 6 years
For Distributors:
Although the deadline for serialization affects manufacturers and repackagers this month, it is important that distributors understand the downstream effects it will have on them and have processes in place to receive PIs. If your company utilizes a repackaging service, make sure that they are aware of the November 27th deadline. Furthermore, stay in contact with your trading partners to ensure that your LUM programs remain compliant as manufacturers will be setting the lowest unit of sale.
What To Expect On November 27, 2023 (Phase II):
Package level requirements for the interoperable, electronic tracing products go into effect, including those relating to:
- Verification of PIs at the package level
- Prompt response to suspect and illegitimate products when found
- Secure tracing at the package level
- This section of the law is self-effectuating—there will be no “final” rule from FDA
DSCSA Timeline
2013
2015
- January 2015: Receive/Pass TH/TI/TS
- January 2015: Transact only with authorized trading partners
2018
- November 27, 2018: Manufacturers & Repackagers Serialize
2019
- November 27, 2019: Distributors Transact Only in Serialized Product
2020
- November 27, 2020: Dispensers Transact Only in Serialized Product
2023
- November 27, 2023: Fully Interoperable
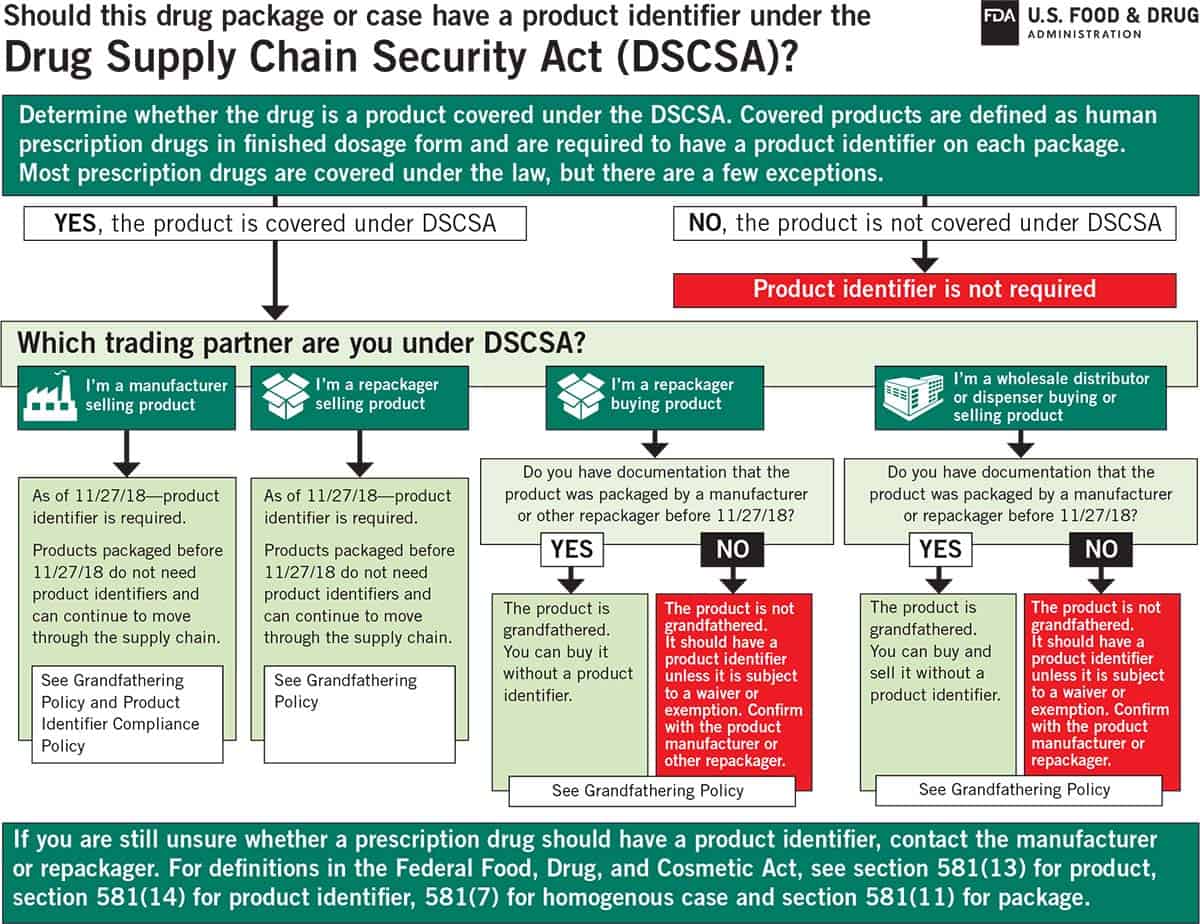
Flowchart
Download As PDF
Helpful Resources
For more information, contact HIDA Government Affairs at HIDAGovAffairs@hida.org.